
User defined models are possible when the standard models do not suffice.

The latter has standard PKPD models ready to use. It is often used in combination with the subroutine package PREDPP. It is a powerful tool which can be used in the analyses of population pharmaokinetic (PK) and pharmacodynamic (PD) data.
#NONMEM COVARIATE ANALYSIS SOFTWARE#
A.Is model analysis software used for fitting and simulation data.
#NONMEM COVARIATE ANALYSIS PDF#
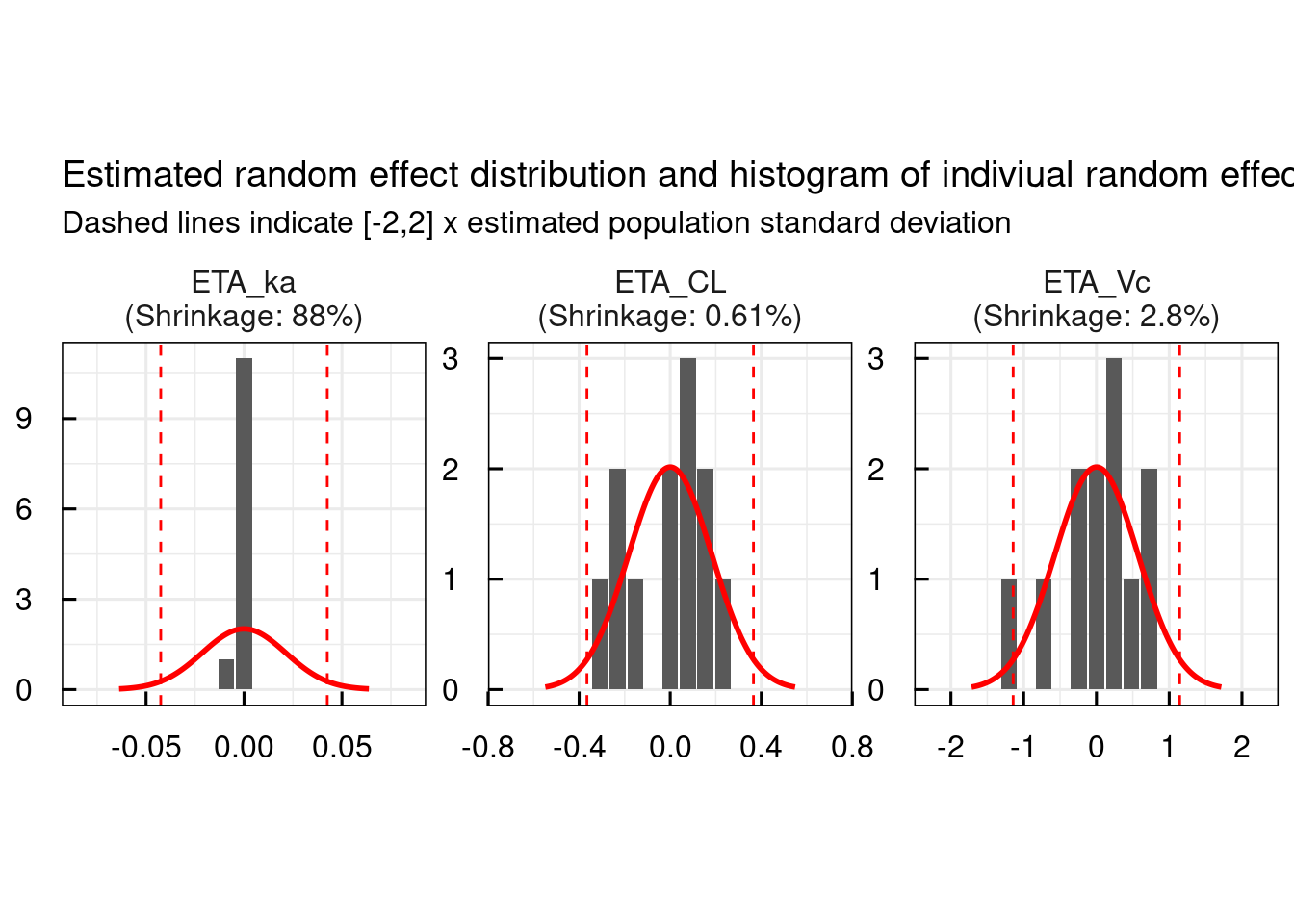
IBook and pdf versions of this material and other PK material is availableĬopyright © 2001-2021 David W. Material on this website should be used for Educational or Self-Study Purposes Only This page was last modified: Monday, 6th Apr 2020 at 8:39 pm Notice the scatter of points above and below the horizontal zero line. Other tabular output would include time, observed and calculated data (not shown).Ī variety of graphical output is also possible including observed versus calculated data, Figure 22.5.6 and weighted residual versus the observed value, Figure 22.5.7.įigure 22.5.6 Observed Data versus Calculated Dataįigure 22.5.7 Weighted Residual versus Concentration These values provide an estimate of the uncertainty in the parameters and the data values. Two ETA values and one EPS1 value are also output in this section. The TH values refer to the THETA parameters described in Figure 22.5.1 for slope (TA) and intercept (TB) of the kel (K) equation and volume, V. The fourth and fifth column input the time and value for each of the two samples and the final column specifies the creatinine clearance for this subject.įigure 22.5.4 Progress during and toward the End of the Iterative Process The next two columns provide the infusion amount and rate for the first of each three data lines. The first column is the subject ID number. The data is input in tabular form as described above in Figure 22.5.1. Estimation limits, tabular and graphical output are specified later in this section. Parameter limits and initial values are specified in this section. The data error or variance equation are defined as the parameter Y.įigure 22.5.2 Analysis and Output Specification for NONMEM The rest of this section describe model parameters K and S1 in terms of parameter (THETAs) and variability terms (ETAs). The data filename and the model subroutine are on the next two lines. Here the data title and data format are specified on the first two lines. The subjects are given a drug by IV infusion and two blood samples were collected.įigure 22.5.1 The Data and Model Specification for NONMEM The user provides control and data files to NMTRAN which produces files required to compile, link and run NONMEM.Īs an example we can consider some data simulated for 'patients' with varying renal functions. The use of NONMEM is aided by the use of NMTRAN, a pre-processing program. The PopPK approach can also be used in cases of data rich sources, such as bioavailability studies, or sparse data information that might be available post-marketing during therapeutic drug monitoring. The output from these analyses may be useful in the Bayesian estimate of clinical pharmacokinetic data as described earlier. NONMEM will allow the analyst to include data from many subjects in one analysis and provides estimates of the best-fit pharmacokinetic parameter values and estimates there variability (standard deviation or vairance) between the subject as well as relationships with covariate values. One of these population pharmacokinetic (PopPK) computer programs is NONMEM.
Fortunately there are data from a large number of subjects and there are a number of computer programs which are capable of analysing these data simultaneously. It is difficult to analyse data separately to determine good estimate of the pharmacokinetic parameters. In these studies the protocol may provide for the collection of only one or two blood samples during various dosage regimens. These subjects may provide a wide range of covariates such as age, weight, sex, genetic characteristics, co-administration of other drugs and various clinical or pathological conditions. Later studies during Phase III involve the adminstration of the NDE to numerous patients who may be expected to have some therapeutic benefit from the NDE.
The data analysis techniques described earlier in this course may be quite useful for these studies. These studies usually involve the collection of numerous blood and other samples from each subject. The early, Phase I studies are typically conducted in healthy volunteers with the objective of determining the disposition charateristics of the NDE. Chapter 22 - Chapter 22 Non-Linear Regression Analysis of Population DataĪnalysis using of Population Data using NONMEMĭuring the development of new drug entities (NDE) the manufacturer will study the disposition of the drug in numerous volunteeers healthy and otherwise.


 0 kommentar(er)
0 kommentar(er)
